Component 1
Structure and function of Adenosine Triphosphate (ATP)
In biological systems, it is chemical energy that makes the changes because chemical bonds must be broken or formed for reactions to happen. Some organisms (like humans) derive their chemical energy from the food they eat and are called heterotrophs. Other organisms (like plants) make their own food in the form of carbohydrates from the process of photosynthesis. They convert light energy into chemical energy (such as glucose) and are called autotrophs.
ATP as an energy carrier
Organisms store chemical energy mainly in the form of carbohydrates and lipids. However, the molecule that makes this energy available to the organism when it is needed is called adenosine triphosphate (ATP). We make and break down about 50 kg of ATP every day but the body only contains about 5g of ATP at any one time, so ATP is not an energy store. 5g of ATP would provide a few seconds supply of energy for a human at rest. The ATP can not be stored so must be made continuously. Cells generating movement or performing actie transport contain many mitochondria, where ATP is synthesised. ATP is sometimes called the "energy currency" inside the cell because ATP releases energy when changes happen in the cell.
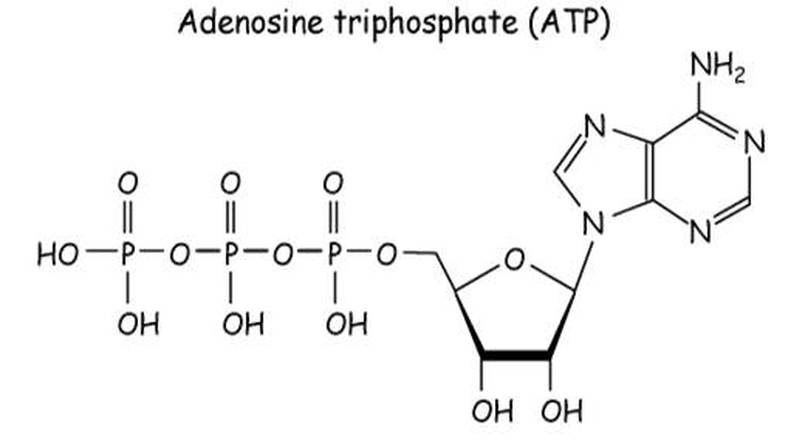
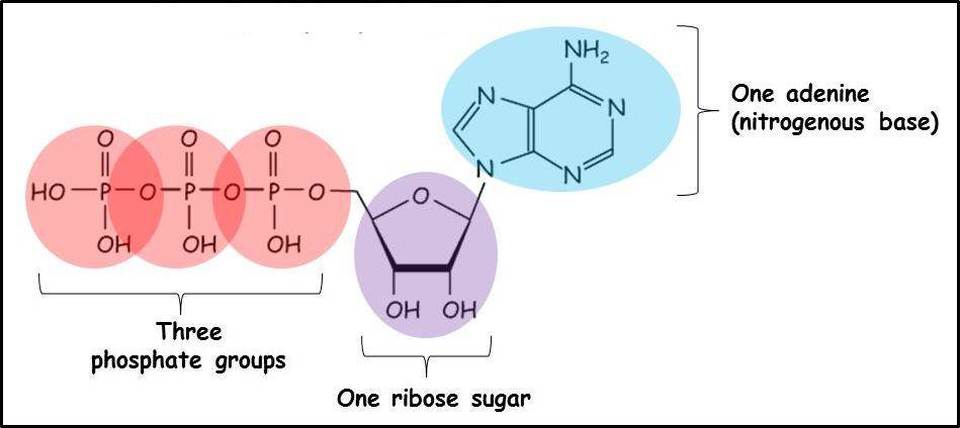
The structure of ATP
ATP is a nucleotide. The intials stand for adenosine triphosphate, which indicates that it contains the nitrogenous base adenine, the pentose sugar ribose and three phosphate groups. These components are linked together into a single molecule through condensation reactions.
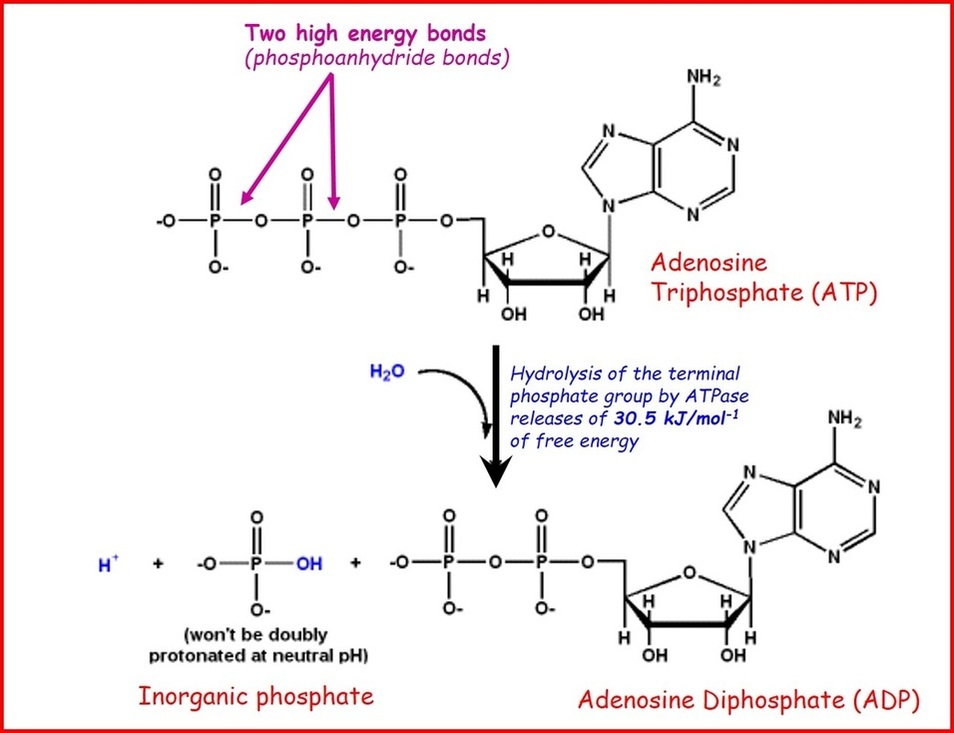
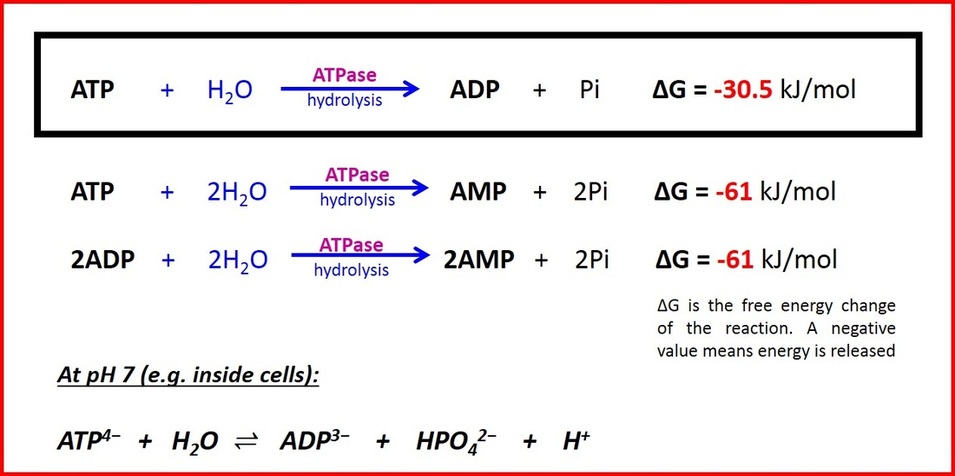
ATP and energy release
When energy is needed inside living cells, the enzyme ATPase hydrolyses the bond between the second and third phosphate group in ATP, removing the third group and leaving only two. The ATP molecule is hydrolsed into adenosine diphosphate (ADP) and an inorganic phosphate ion with the release of chemical energy. Every mole of ATP that is hydrolysed releases 30.6kJ when the bond is broken. A reaction that releases energy, such as ATP hydrolysis, is an exergonic reaction.